Clinical Trials Should Be Scientifically Sound and Described in a
A trial should be conducted in compliance with the protocolthat has received. Clinical trials should be scientifically sound described in a clear and detailed protocol.
A Practical Overview Of Patient Centric Trials
26 A trial should be conducted according to the protocol having received prior IRB approval.

. A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics. A trial should be conducted in compliance with the protocol that has received prior Institutional Review Board IRBIndependent Ethics Committee IEC approval. Good quality trials Clinical trials should be scientifically sound and be described in a clear detailed protocol.
Despite the appearances however it is contentious that placebo-controlled trials PCTs are inherently deceptive towards participants. A phase of research to describe clinical trials that gather preliminary data on whether a drug works. The use of placebo injections violated international ethical guidelines.
A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC approvalfavourable opinion. 26 A trial should be conducted in compliance with the protocol that has received prior independent ethics committee approval 27 The medical care given to and medical decisions made on behalf. A trial should be conducted in compliance with the protocol that has received a favourable.
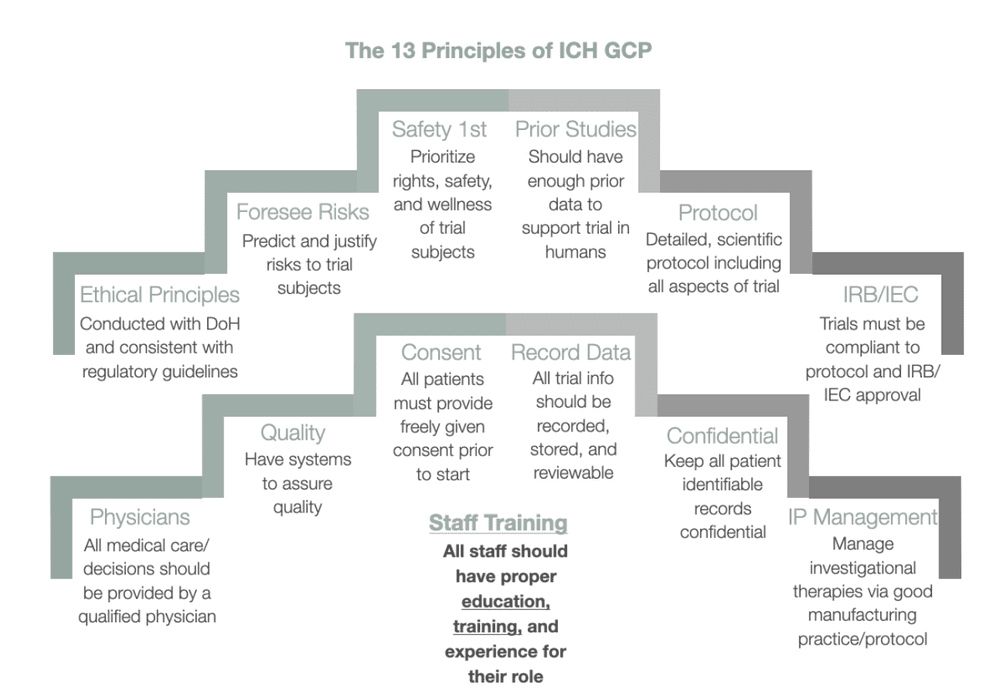
Clinical trials should be scientifically sound and described in a clear detailed protocol. Clinical trials should be conducted in accordance with the ethical principles that are consistent with GCP and the applicable regulatory requirements and that have their origin in the Declaration of _____. Clinical trials should be scientifically sound and described in a clear detailed protocol.
13 Principles of GCP 13 Principles of GCP 5. We may at any time request. Compliance with the study protocol A trial should be conducted in compliance with the protocol that has received prior.
The saltwater was not the best intervention. In other words answers to the research question should contribute to scientific understanding of health or improve our ways of preventing treating or caring for people with a given disease to justify exposing participants to the risk and burden of research. Clinical trials should be scientifically sound and described in a clear detailed protocol.
25 Clinical trials should be scientifically sound and described in a clear detailed protocol. The clinical trial must have a written protocol that describes a scientifically sound study that has been approved by all relevant institutional review boards IRBs before participants are enrolled in the trial. The clinical study must be approved by an Institutional Review Board IRB a committee that has been formally designated to approve monitor and review biomedical and behavioral research.
Clinical trials should be scientifically sound and described in a clear detailed protocol. A trial should be conducted in compliance. Clinical trials should be scientifically sound and described in a clear detailed protocol.
There was no compelling scientific reason for placebo use exposing subjects to risk of a potentially fatal infection. Clinical trials should be scientifically sound and described in a clear detailed protocol. 10 13 Principles of GCP cont.
25 Clinical trials should be scientifically sound and described in a clear detailed protocol. 26 A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC. In accordance with the TGA Note for Guidance on Good Clinical Practice CPMPICH13595 Section 25 clinical trials should be scientifically sound and described in a clear detailed protocol.
Patients on the placebo arm of a clinical trial must be made to believe they are receiving a working treatment even though they are not for the placebo effect to play a role at all. Clinical trials should be scientifically sound and described in clear detailed protocol. Product should be adequate to support the proposed clinical trial.
A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC approvalfavourable opinion. 25 Clinical trials should be scientifically sound and described in a clear detailed protocol. Clinical trials should be scientifically sound and described in a clear detailed protocol.
Clinical trials should be scientifically sound and described in a clear detailed protocol. A trial should be conducted in compliance with the protocol that has received prior Institutional Review Board IRB Independent Ethics Committee IEC approvalfavorable opinion. Clinical trials should be scientifically sound and described in a clear detailed protocol.
Clinical trials should be scientifically sound and described in a clear detailed protocol E6 25 Each individual involved in conducting a trial should be qualified by education training and experience to perform his or her respective tasks E6 28 Classified as public by the European Medicines Agency. 27 The Subjects medical care should always be the responsibility of a qualified physician. Clinical trials should be scientifically sound and clearly described in the study protocol.
A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC approvalfavorable opinion. The Principles of GCP continued. And there was an active treatment available to design a scientifically and ethically sound clinical trial.
Clinical trials should be scientifically sound and described in a clear detailed protocol. Compliance with the study protocol A trial should be conducted in compliance with the protocol that has received ethics committee and MHRA approval. Clinical trials are conducted according to a plan called a protocol which describes.
A study should be designed in a way that will get an. A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC approvalfavorable opinion. Product should be adequate to support the proposed clinical trial.
What the researchers hope to learn from the study. The clinical trial should be conducted in compliance with the study protocol.
The Importance Of Ich Gcp Clinical Research Certification

Thwarting Subjectivity In Clinical Trials Fierce Biotech

Are Clinical Trials A Last Resort Doctors Discuss The Benefits And Risks For Patients Medifind
(135).jpg)
No comments for "Clinical Trials Should Be Scientifically Sound and Described in a"
Post a Comment